Study Guide
Chronic Osteomyelitis
Key Points:
- Chronic osteomyelitis is an infection of bone with a duration of symptoms > 4 weeks.
- The most common symptom of chronic osteomyelitis is pain. The clinical presentation of chronic osteomyelitis is often indolent, and patients rarely appear acutely ill.
- ESR and CRP values are typically elevated and are used to evaluate response to treatment. It is important to keep chronic osteomyelitis within the differential diagnosis but also imperative to rule out other possible etiologies such as malignancy.
- The most common organism involved in osteomyelitis is Staphylococcus aureus.
- X-rays can show osseous changes in the setting of chronic osteomyelitis, but MRI remains the most critical imaging modality.
- Little literature exists regarding the optimal treatment strategy for chronic osteomyelitis; however, the gold standard includes aggressive surgical debridement followed by 4-6 weeks of antibiotic therapy.
- The most common complications include abscess, fistula, and sinus tract formation.
Description:
Osteomyelitis is defined as an infection of the bone and can be associated with adjacent soft tissue or intra-articular joint infections. Osteomyelitis in the pediatric population can be classified by patient age (neonate, infant, etc.) or by the duration of symptoms. Acute osteomyelitis is defined by a period of symptoms of less than two weeks; osteomyelitis is considered chronic when symptoms have been present for longer than four weeks. (Dormans,1994; Matzkin, 2005)The metaphysis is the most common location of osteomyelitis in the pediatric population, followed by the diaphysis and rarely the epiphysis. (Matzkin, 2005) The reasoning for this is due to the vascular anatomy of skeletally immature bone. Both the metaphysis and diaphysis are fed by the same nutrient arteries while the epiphysis has an independent vascular supply; after approximately 12-18 months of age, no transphyseal anastomoses remain. The sinusoidal lakes connecting the arterial and venous systems within the bone are low flow areas that allow for the potential accumulation of micro-organisms. (Dormans, 1994; Schuppen, 2012) While the physis serves as a successful barrier against the spread of infection to the epiphysis in the vast majority of cases of acute osteomyelitis, in chronic osteomyelitis, transphyseal involvement is relatively common. (Dormans, 1994)
Once micro-organisms begin to pool within the sinusoidal lakes, subsequent thrombosis of the surrounding arterioles and venules decreases immunologic cell delivery to the area and allows further propagation of the infection. If left untreated, such as in the setting of chronic osteomyelitis, the continued growth of the bacteria will cause periosteal elevation. The periosteum will create a new outer layer of bone known as the involucrum, while the underlying necrotic layer of cortical bone becomes the sequestrum. With time, fistulas and draining sinus tracts can also develop. (Dormans, 1994)
Epidemiology:
Osteomyelitis within the pediatric population is a rare condition with a reported annual incidence of 3-20 per 100,000 children, with the majority of these cases representing acute osteomyelitis. (Otani, 2019; Schuppen, 2012; Street, 2015) Pediatric osteomyelitis is approximately twice as common in males compared to females (Matzkin, 2005; Schuppen 2012). Interestingly, Pacific Islanders have a well-reported greater incidence and severity of osteomyelitis. (Matzkin, 2005; Stone, 2016)The most frequently identified organism in pediatric osteomyelitis is Staphylococcus Aureus. (Geurts, 2017; Canavese, 2016; Matzkin, 2005; Schuppen, 2012). Other common pathogens include Escherichia coli, Group B Streptococcus, Bacillus subtilis, Kingella kingae, Proteus, and Pseudomonas. (Geurts, 2017) Methicillin-resistant Staphylococcus aureus (MRSA) infections, while not common in the pediatric population, are increasing in prevalence and are more commonly associated with abscess formation and myositis. (Schuppen, 2012) Infections can also be polymicrobial, and in the chronic setting, a specific pathogen may never be identified. (Geurts, 2017)
The tibia is the most commonly involved bone, followed by the femur and humerus. (Geurts, 2017; Stone, 2016) Most cases are localized to a single site; however, infants and neonates have reported rates of polyostotic involvement in up to 7% and 20% of cases, respectively. (Schuppen, 2012)
Identified risk factors for the development of osteomyelitis include direct trauma, sickle cell disease, septic arthritis, immunodeficiency, sepsis, chronic indwelling catheters, and chronic vascular access lines. The risk of developing pediatric osteomyelitis decreases with increasing age, presumably because neonates have an underdeveloped immune system. (Schuppen, 2012) Younger children are also less able to communicate their symptoms, which can lead to a delay in diagnosis. (Otani, 2019; Schuppen, 2012)
Clinical Findings:
As an infectious and inflammatory process, the clinical presentation of osteomyelitis can often include fever, localized pain, altered weight-bearing, and/or general reluctance to use the affected limb. Patients typically will have at least one, but not all, of these common symptoms; the most consistently reported symptom in chronic osteomyelitis is pain. (Schuppen, 2012; Stone, 2016) While patients with acute osteomyelitis may be toxic appearing, those with chronic osteomyelitis are typically less ill; this can pose an even more significant challenge in evaluating young patients who are nonverbal. (Dormans, 1994) In the unique patients with sickle cell disease, the clinical presentation can be indistinguishable from osteonecrosis or a sickle cell crisis, and further workup with imaging is often required. (Schuppen, 2012)Many patients also present to the orthopaedic surgeon for evaluation after initially being evaluated by their pediatrician and may have been recently given antibiotics for a presumed different diagnosis, such as an ear infection or urinary tract infection. This can result in masking of the signs and symptoms of osteomyelitis. (Dormans, 1994; Otani, 2019) Given the low incidence within the pediatric population, paired with the variable and often obscure clinical presentation, nearly half of bacterial osteomyelitis cases are initially misdiagnosed, of which 30% may be initially misdiagnosed by the orthopedic surgeon (Otani, 2019) Therefore, it is imperative to obtain a thorough history and to include infectious processes within the differential diagnosis.
In addition to imaging studies (discussed below), obtaining appropriate laboratory tests is an important step in the diagnostic evaluation of osteomyelitis. At a minimum, patients being evaluated for possible osteomyelitis should have a complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and blood cultures drawn. Even in symptomatic patients, it is important to be aware that lab values may be normal. (Copley, 2009; Stone, 2016) There is some debate regarding whether ESR or CRP is more sensitive in the setting of chronic osteomyelitis; however, both are frequently utilized to evaluate response to treatment, so having a baseline lab value is important. As an acute phase reactant, CRP values can demonstrate significant change within a matter of days, making it useful in assessing acute response to treatment. In contrast, ESR can take weeks to demonstrate significant change and is more useful in assessing the efficacy of long term treatment. (Canavese, 2016; Copley, 2009; Matzkin, 2005; Stone, 2016) It is important to note that although elevations in CRP and/or ESR are common, the degree of elevation of these inflammatory markers has not been correlated with the severity of an infection. (Matzkin, 2005)
Imaging Studies:
While plain radiographs only have a reported sensitivity of 25-75% and specificity of 75-83%, they should still be included in the initial workup. Periosteal reactions can be noted within a few days, and by three weeks, lytic lesions and endosteal scalloping can be appreciated. (Figure 1) Bony destruction secondary to chronic osteomyelitis can appear as either a lucency or as a permeative lesion and, therefore should direct orthopaedists to consider a differential diagnosis including not only infection but also Ewing’s sarcoma, Langerhans Cell Histiocytosis, and possible metastasis. (Dormans, 1994; Schupppen, 2012)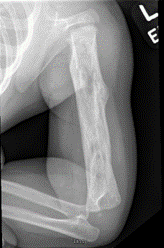 Figure 1 (left): Chronic osteomyelitis of the humerus in a 10 month old child. Note the lytic lesions and perisoteal reaction.
Figure 1 (left): Chronic osteomyelitis of the humerus in a 10 month old child. Note the lytic lesions and perisoteal reaction.Ultrasound is another imaging modality that can be utilized in the evaluation of chronic osteomyelitis. As always, the sensitivity and specificity of ultrasound is operator dependent but has been reported to range from 46-74% and 63-100%, respectively. Ultrasound does not provide adequate visualization of the actual bone but can be helpful in evaluating associated processes such as abscesses, fistulas and sinus tracts, and surrounding soft tissue edema. If severe cortical defects exist, these may be visualized with ultrasound. (Schuppen, 2012)
While computed tomography (CT) imaging plays little role in the assessment of acute osteomyelitis, it can allow for better visualization of cortical destruction and sequestrum in the chronic setting. (Schuppen, 2012) The obvious disadvantage of obtaining CT scans, especially in the pediatric population, is the associated high radiation burden, although modern CT protocols may allow for a low-radition CT. CT scan may be more readily available and quicker to obtain, without the need for sedation.
In the setting of acute and chronic osteomyelitis, magnetic resonance imaging (MRI) is the most important imaging modality. In addition to sparing patients from radiation exposure, MRI allows for excellent evaluation of both bony and surrounding soft tissue involvement. Osteomyelitis demonstrates a low signal intensity on T1 sequences and high signal intensity on T2 and STIR sequences. The reported sensitivity and specificity of MRI imaging in osteomyelitis is also superior to other imaging modalities at 82-100% and 75-99%, respectively. (Schuppen, 2012) Unfortunately, obtaining MRI in an expedient manner may be difficult, especially if the patient requires sedation. Often in the setting of chronic osteomyelitis and a physiologically stable patient, MRIs can be done on a semi-elective basis. In the sick or septic child fast sequence MRI scans can often be done without sedation to assess the bone and joint to assist in making urgent treatment decisions such as the need for surgical debridement.
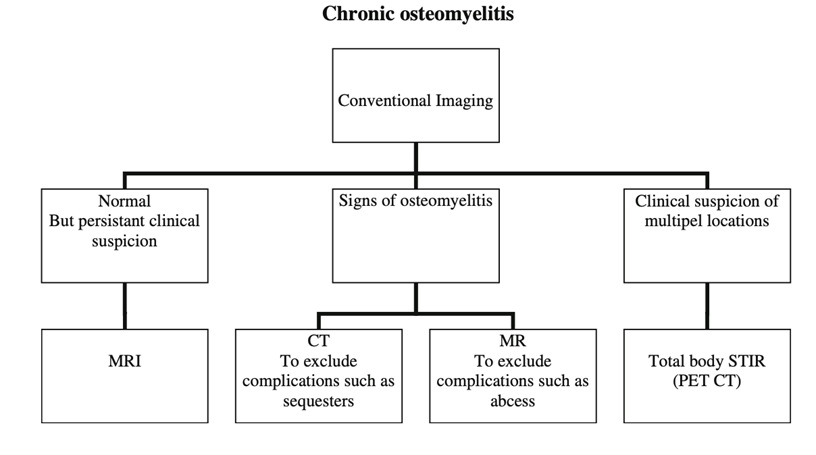 Figure 1 (right): Proposed Imaging Strategy from Schuppen, 2012
Figure 1 (right): Proposed Imaging Strategy from Schuppen, 2012
Treatment:
While acute osteomyelitis can often be treated successfully without surgical intervention, the presence of sequestrum, sinus tracts, fistulas, and abscesses associated with chronic osteomyelitis requires a combination of surgical and medical therapy to eradicate the infection. A meta-analysis of studies evaluating the treatment of osteomyelitis failed to provide enough evidence to declare the most effective treatment protocol. Still, it did demonstrate that antibiotics, in addition to surgery, yielded the highest rates of recovery. (Geurts, 2017)In the setting of chronic osteomyelitis antibiotics should be held until intra-operative cultures are obtained, and a known pathogen is identified and used to tailor treatment. If the severity of the patient’s medical condition prevents delaying the initiation of antibiotics, broad-spectrum coverage for common pathogens should be initiated. Should any concern exist regarding the diagnosis of osteomyelitis, a biopsy may be required before surgical debridement to ensure appropriate treatment is being provided for infection and not a malignancy.
After intraoperative cultures have been obtained, surgical intervention should consist of sharp debridement of all necrotic appearing tissue, sequestrectomy, abscess drainage, removal of any hardware or other foreign bodies, and large-volume irrigation. (Canavese, 2016; Copley, 2009) If needed, a cortical window can be created under fluoroscopic guidance to aid in accessing the sequestrum or abscesses; care should be taken to avoid damaging the physis. (Copley, 2009) In the setting of large areas of bony involvement with resultant bony defects after debridement, the Masquelet technique for staged treatment has also been proposed. (Canavese, 2016)
After surgical debridement and the identification of a known pathogen, IV antibiotic therapy should be initiated. The ideal length of IV antibiotics is debated, however, the commonly accepted practice is to continue parenteral antibiotics until the patient has shown clinical improvement and inflammatory markers have begun to normalize. (Canavese, 2016; Copley, 2009) At that time, the transition to oral antibiotics can occur.
The optimal length of oral antibiotic therapy is also debated, but common practice includes a total of at least 4-6 weeks of antibiotic therapy. (Copley, 2009) Some studies suggest that a longer duration of therapy may be required if adjacent joint involvement is noted. (Matzkin, 2005) At the time of scheduled completion of antibiotics, ESR and CRP should be obtained; if these labs remain elevated, an additional 2-3 weeks of oral antibiotics should be given. If inflammatory markers have not normalized after 12 weeks of antibiotic therapy, repeat MRI should be considered to assess for continued evidence of infection. (Copley, 2009)
Complications:
The most common complications associated with chronic osteomyelitis are intraosseous abscess, fistula, or sinus tract formation. Most associated abscesses occur within the metaphysis. When an intra-osseous abscess forms in the metaphysis, this is referred to as a Brodie’s abscess. Abscesses can also occur in the surrounding soft tissues. (Dormans, 1994; Schuppen, 2012)
In the setting of transphyseal involvement, there is risk of growth arrest secondary to physeal damage. (Dormans, 1994; Stone, 2016) In the setting of large areas of involvement, there is an increased risk for associated pathologic fracture from either the patient’s normal weight-bearing load or secondary to aggressive debridement.
As in all cases of infection, the risk of developing non-healing wounds should also be considered when treating chronic osteomyelitis. Patients with systemic illnesses such as diabetes or autoimmune disease are at higher risk for developing chronic osteomyelitis at baseline and are also at higher risk for developing subsequent wound complications. Depending on the location and severity of chronic wounds, amputation may be ultimately required.
Related Videos:
References:
1. Canavese F, Corradin M, Khan A, Mansour M, Rousset M, Samba A. Successful treatment of chronic osteomyelitis in children with debridement, antibiotic-laden cement spacer, and bone graft substitute. European Journal of Orthopaedic Surgery & Traumatology. 2016;27(2):221-228. doi:10.1007/s00590-016-1859-7.2. Copley LAB. Pediatric Musculoskeletal Infection: Trends and Antibiotic Recommendations. Journal of the American Academy of Orthopaedic Surgeons. 2009;17(10):618-626. doi:10.5435/00124635-200910000-00004.
3. Dormans JP, Drummond DS. Pediatric Hematogenous Osteomyelitis: New Trends in Presentation, Diagnosis, and Treatment. Journal of the American Academy of Orthopaedic Surgeons. 1994;2(6):333-341. doi:10.5435/00124635-199411000-00005.
4. Geurts J, Hohnen A, Vranken T, Moh P. Treatment strategies for chronic osteomyelitis in low- and middle-income countries: systematic review. Tropical Medicine & International Health. 2017;22(9):1054-1062. doi:10.1111/tmi.12921.
5. Matzkin EG, Dabbs DN, Fillman RR, Kyono WT, Yandow SM. Chronic osteomyelitis in children: Shriners Hospital Honolulu experience. Journal of Pediatric Orthopaedics B. 2005;14(5):362-366. doi:10.1097/01202412-200509000-00009.
6. Otani Y, Aizawa Y, Hataya H, Horikoshi Y. Diagnostic errors in pediatric bacterial osteomyelitis. Pediatrics International. 2019;61(10):988-993. doi:10.1111/ped.13979.
7. Schuppen JV, Martine M. A. C. Van Doorn, Rijn RRV. Childhood osteomyelitis: imaging characteristics. Insights into Imaging. 2012;3(5):519-533. doi:10.1007/s13244-012-0186-8.
8. Stone B, Street M, Leigh W, Crawford H. Pediatric Tibial Osteomyelitis. Journal of Pediatric Orthopaedics. 2016;36(5):534-540. doi:10.1097/bpo.0000000000000472.
9. Street M, Puna R, Huang M, Crawford H. Pediatric Acute Hematogenous Osteomyelitis. Journal of Pediatric Orthopaedics. 2015;35(6):634-639. doi:10.1097/bpo.0000000000000332.
10. Zhao Y, Ferguson PJ. Chronic Nonbacterial Osteomyelitis and Chronic Recurrent Multifocal Osteomyelitis in Children. Pediatric Clinics of North America. 2018;65(4):783-800. doi:10.1016/j.pcl.2018.04.003.

